
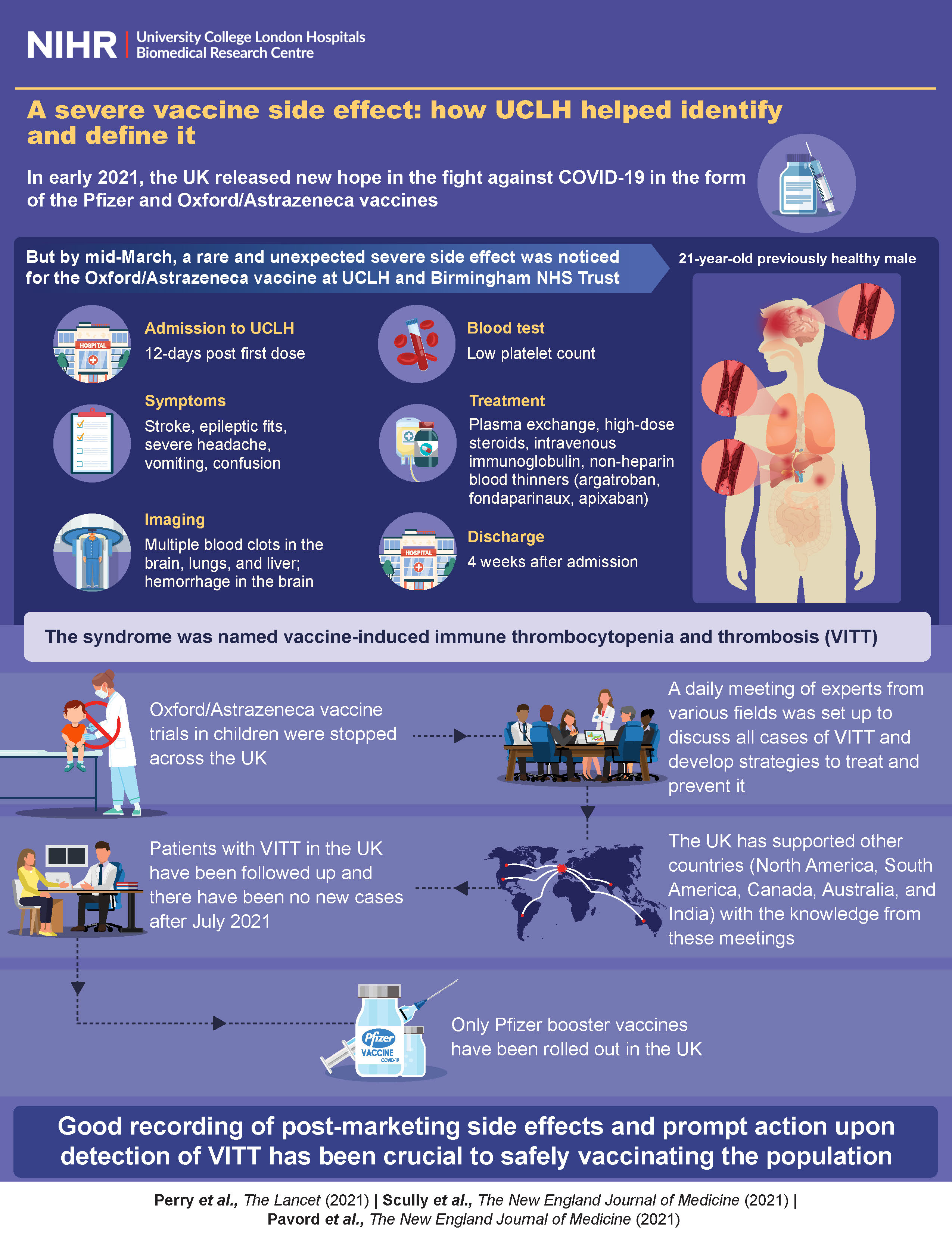
AstraZeneca has for the first time officially acknowledged in court documents that its Covid-19 vaccine, sold as Covishield in India and Vaxzevria elsewhere, could cause a rare side-effect called Thrombosis Thrombocytopenia Syndrome (TTS). This comes after a UK-based man, Jamie Scott, was left with a permanent brain injury due to blood clotting and low platelet count after receiving the vaccine. AstraZeneca is now facing 51 legal cases in the UK with victims seeking up to 100 million British pounds in compensation. The company, however, has contested these claims but has accepted that the vaccine can cause TTS in very rare cases.
AstraZeneca Vaccine Linked to Rare Blood-Clotting Side Effect
Background:
AstraZeneca, a British-Swedish pharmaceutical company, developed a COVID-19 vaccine called Vaxzevria (also known as Covishield in India). The vaccine utilized an adenovirus vector technology to carry genetic material of the virus into cells, triggering an immune response.
Court Acknowledgement of Blood-Clotting Side Effect:
Recently, AstraZeneca acknowledged in court documents that its COVID-19 vaccine could cause a rare side effect called Thrombosis Thrombocytopenia Syndrome (TTS). TTS is characterized by the formation of blood clots in the brain and a low platelet count.
Legal Cases and Compensation Claims:
Following reports of TTS cases linked to the AstraZeneca vaccine, a UK-based man, Jamie Scott, filed a lawsuit alleging he suffered a permanent brain injury after receiving the shot. The case has sparked 51 legal claims in the UK, with victims seeking up to 100 million British pounds in compensation.
AstraZeneca's Response:
AstraZeneca has contested the claims of the victims, maintaining that the vaccine is safe and effective. However, the company has accepted that TTS can occur in very rare cases.
Top 5 FAQs and Answers
1. What is Thrombosis Thrombocytopenia Syndrome (TTS)?
TTS is a rare blood-clotting disorder that can occur after vaccination with the AstraZeneca COVID-19 vaccine. It involves the formation of blood clots in the brain and a low platelet count.
2. How common is TTS?
TTS is a very rare side effect of the AstraZeneca vaccine, estimated to affect around 1 in 100,000 recipients.
3. What are the symptoms of TTS?
Symptoms of TTS include severe headache, blurred vision, slurred speech, weakness or numbness on one side of the body, difficulty breathing, and chest pain.
4. What is the treatment for TTS?
Treatment for TTS typically involves administering intravenous immunoglobulin and anticoagulants to prevent further blood clots.
5. Is the AstraZeneca vaccine safe for use?
The AstraZeneca vaccine is safe and effective in preventing severe COVID-19 illness and death. However, individuals with a history of blood-clotting disorders or those at high risk of TTS should consult with their healthcare provider before receiving the AstraZeneca vaccine.

A remote monitoring camera operated by the US Geological Survey captured stunning visuals of the recent volcanic eruption at Hawaii's Kilauea Volcano. The footage showed lava fountains up to 100 feet high and the raw power of nature as the lava eventually engulfed the camera. This eruption, known as Episode 38, was the latest in a series of eruptions that have been occurring since December last year. However, according to USGS, another episode could take place in the near future.

Indian Prime Minister Narendra Modi virtually inaugurated Skyroot Aerospace's new Infinity Campus in Hyderabad and unveiled their first orbital launch vehicle, the Vikram-I. During the event, PM Modi praised India's advancements in space technology and spoke about the importance of private companies like Skyroot in driving innovation in the space sector. The Infinity Campus, equipped with state-of-the-art facilities, has the capacity to produce one rocket per month, marking a significant milestone in India's private space manufacturing capability. Skyroot Aerospace, founded by former ISRO engineers, has quickly become a prominent player in India's growing space industry, with the successful launch of Vikram-S, the country's first privately built sub-orbital rocket.

We all experience changes in our mood, whether it's feeling happy and content or irritated and moody. But what are the underlying factors that contribute to these changes? This article from Medindia explores the top 10 things that can affect our mood, from physical health to environmental factors. It also provides tips on how to avoid these mood-altering triggers and maintain a positive state of mind. With a focus on promoting overall well-being, Medindia's policies align with the UN's Sustainable Development Goals, making it a reliable source of information for health and wellness.

A recent report by Public Health Scotland has shown a steep increase in flu cases and hospitalizations in Scotland. The numbers have more than doubled from the previous week, with a higher intensity observed in younger age groups. Experts are warning of a long flu season and a new variant of the illness that is spreading more easily. Health Secretary Neil Gray has assured the public that there are enough doses of flu vaccine available in the country.

The observation of National Pollution Control Day on 2 December serves as a timely reminder of India's struggle with escalating pollution levels. The recent years have seen a sharp increase in toxic particles and hazardous emissions, causing severe health issues and environmental damage. The ongoing pollution emergency calls for more stringent regulations, better urban planning, and increased public engagement to mitigate the crisis.

As World AIDS Day approaches, conversations around HIV prevention in India are becoming more open and informed. In particular, there is growing interest in PrEP (pre-exposure prophylaxis), a medicine that offers strong protection against HIV when used correctly. With rising awareness and more accessible sexual-health services, doctors are seeing a steady rise in patients asking about PrEP as a proactive health choice. This signals a shift towards informed prevention and a stigma-free dialogue surrounding HIV.

ISRO has been making continuous efforts to establish contact with the Vikram lander and Pragyan rover, which were put into sleep mode earlier this month, ahead of the lunar night. However, the prolonged spell of cold weather conditions, reaching up to -150 degrees Celsius, has made it difficult for them to wake up. With the sunrise on the Moon's south polar region and their solar panels believed to be optimally charged now, ISRO is hoping to revive the lander and rover and continue with their experiments and studies. The latest update from ISRO is that the plan to reactivate them has been delayed to September 23 due to the extreme lunar weather conditions.

Monsoon season may bring romantic vibes, but it's also a nightmare for contact lens wearers. Rainwater contains bacteria and pollutants that can cause eye infections, especially when wearing contact lenses. Ophthalmologists recommend using glasses instead and practicing good hygiene to avoid irritation and infection.

India's first human spaceflight mission, Gaganyaan, is one step closer to reality as ISRO successfully tested the main parachutes for the mission's Crew Module. The test, conducted at the Babina Field Firing Range in Uttar Pradesh, is part of the qualification process for the Gaganyaan parachute system. The system, which includes 10 parachutes of different types, is designed to ensure the safe and stable descent of astronauts returning to Earth. This milestone test marks a crucial step forward for India's ambitious space exploration goals.

As World Pneumonia Day is observed on November 12, experts are drawing attention to the dangerous link between air pollution and respiratory illnesses. In India, the post-Diwali smog adds to the already high levels of pollution, increasing the risk of pneumonia, particularly among vulnerable populations. While outdoor air pollution is often blamed, experts emphasize that poor indoor air quality also plays a significant role in triggering and worsening respiratory infections. Health professionals are urging for better air quality regulations and precautions to prevent this deadly connection between pollution and pneumonia.