
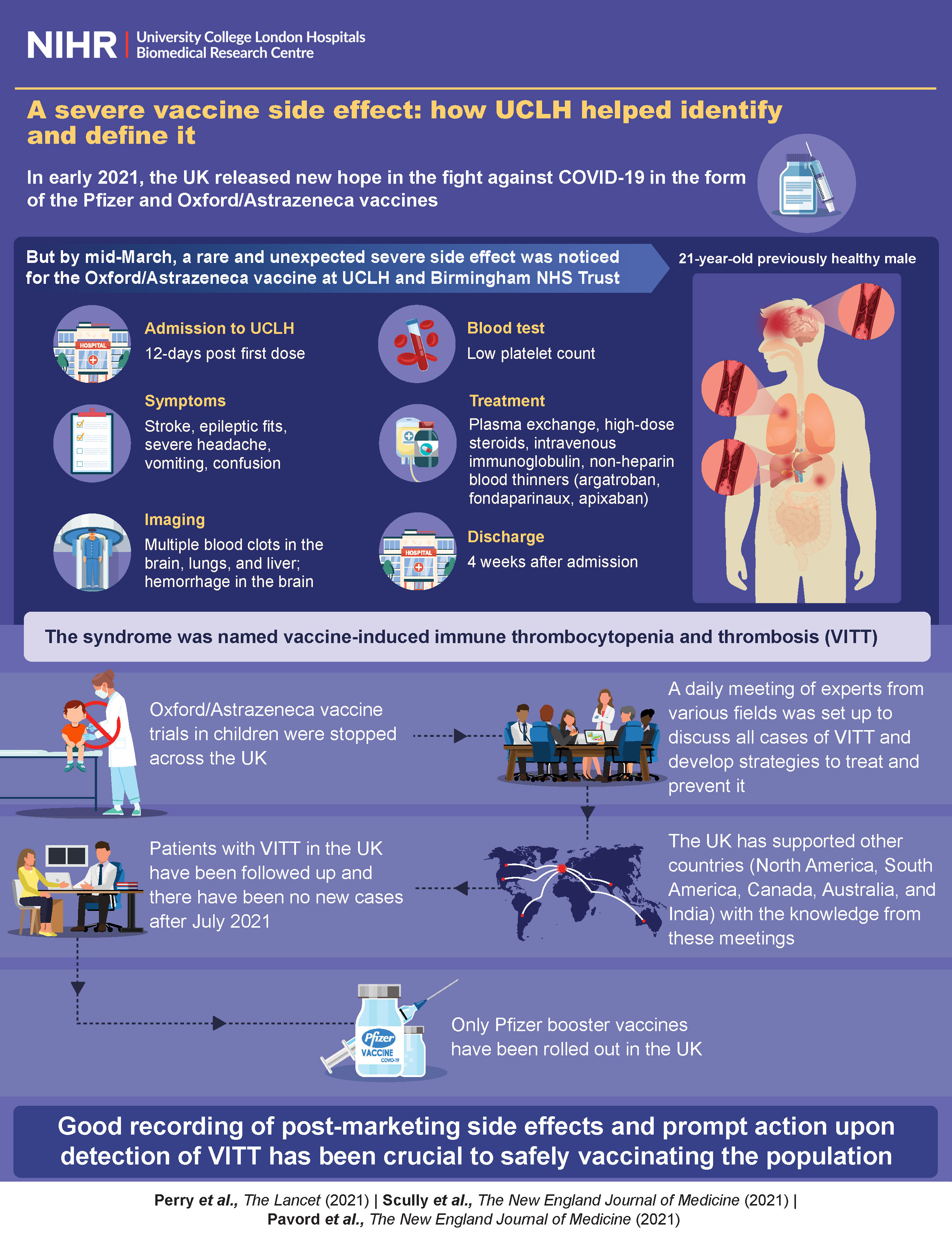
The makers of the Covishield vaccine, AstraZeneca, have acknowledged for the first time that the vaccine may lead to a rare side effect called Thrombosis with Thrombocytopenia Syndrome (TTS). This comes as the company is being sued in a class action and could result in a significant legal payout. Public health experts explain that TTS is a rare condition that has been observed as a potential adverse effect of certain COVID-19 vaccines, including Covishield. They also stress the importance of early detection and treatment of TTS for recovery, and believe that AstraZeneca's admission could lead to enhanced safety monitoring and regulatory oversight for vaccines.
Covishield Vaccine Raises Concerns with Rare Blood Clot Syndrome
Background
Covishield is a COVID-19 vaccine developed by the Serum Institute of India and licensed by AstraZeneca. It is widely used in many countries around the world, including India, Brazil, Argentina, and the United Kingdom.
Thrombosis with Thrombocytopenia Syndrome (TTS)
In recent months, concerns have emerged about the potential link between Covishield and a rare side effect called Thrombosis with Thrombocytopenia Syndrome (TTS). TTS is a condition characterized by blood clots in unusual locations accompanied by low platelet counts.
AstraZeneca Acknowledges Link
On April 7, 2022, AstraZeneca acknowledged for the first time that its vaccine may lead to TTS. This admission came after the company was sued in a class action lawsuit for allegedly failing to adequately warn about the risk of this side effect.
Public Health Experts React
Public health experts have stressed that TTS is a rare condition, but its association with certain COVID-19 vaccines, including Covishield, has been observed. They emphasize the importance of early detection and treatment of TTS for recovery.
AstraZeneca's admission of the potential link between its vaccine and TTS is expected to lead to enhanced safety monitoring and regulatory oversight for vaccines.
Top 5 FAQs and Answers
1. What is Thrombosis with Thrombocytopenia Syndrome (TTS)?
TTS is a rare condition that involves the formation of blood clots in unusual locations, combined with a low platelet count. It can be life-threatening if not treated promptly.
2. Is TTS associated with all COVID-19 vaccines?
TTS has been observed in association with certain COVID-19 vaccines, including the AstraZeneca vaccine. However, the risk is extremely rare, affecting only a tiny fraction of people who receive the vaccine.
3. What are the symptoms of TTS?
Symptoms of TTS can include sudden onset of severe headache, blurred vision, chest pain, shortness of breath, and swelling or pain in the legs. If you experience any of these symptoms after receiving a COVID-19 vaccine, seek medical attention immediately.
4. What is the treatment for TTS?
TTS is treated with anticoagulants (blood thinners) and intravenous immunoglobulin (IVIG) to manage the blood clotting and platelet deficiency, respectively. Early diagnosis and treatment are critical for recovery.
5. Is it safe to receive the Covishield vaccine?
The Covishield vaccine is safe and effective for most people. The risk of TTS is extremely low, and the benefits of vaccination far outweigh the potential risks. It is important, however, to be aware of the potential side effects and to seek medical attention if you experience any unusual symptoms after vaccination.

In an effort to fight the ongoing air pollution crisis, Delhi conducted its first-ever official cloud seeding operation led by IIT Kanpur. The operation involved a small aircraft dispersing specially designed chemical flares into the atmosphere to create rain. While experts say rainfall could occur within 15 minutes to 4 hours, the actual timeframe depends on various factors such as wind direction and moisture content. If successful, the government plans to continue the operation in the coming days.

In the quest for stronger, luscious hair, we often overlook the importance of nurturing the roots. Fortunately, Ayurveda has long stressed the significance of this practice, which has now been backed by modern science. Studies have shown that herbs like Bhringraj and Amla can activate hair follicles, promoting new growth and delaying greying. Fenugreek, Neem, Hibiscus, and Ashwagandha are also found to be beneficial in strengthening and nourishing the scalp, resulting in thicker and healthier hair.

A college student shares her personal journey of becoming a vegetarian, despite facing challenges and health concerns. She then delves into an ethics class she took, where the concept of marginal cases were discussed. Following an article by philosophy professor Alastair Norcross, she concludes that even though individual action may seem insignificant, refusing to consume factory-farmed meat holds moral significance due to the potential to prevent immense suffering for animals.

On October 24, the global community commemorates World Polio Day to honor the legacy of Dr. Jonas Salk and the efforts of countless individuals and organizations in the fight against polio. This highly contagious and potentially deadly disease, once a widespread epidemic, is now largely preventable thanks to the development of a life-saving vaccine. India's successful eradication of polio serves as a testament to the importance of strong vaccination programs and collaborations in public health initiatives.

As winter arrives in India, so does the hazardous air pollution. Delhi NCR's AQI has already crossed the 400 mark, making it crucial to invest in air purifiers, especially after Diwali. Dyson, Qubo, HomePure, and Philips have launched high-quality air purifiers with advanced features to tackle different types of pollutants and create cleaner indoor air. With prices ranging from Rs 5,000 to Rs 1 lakh, these purifiers are a practical and timely purchase for a healthier living.

In a recent family vlog, Indian celebrity couple Shoaib Ibrahim and Dipika Kakar shared their "natural" hair care routine for their son, using a homemade mask made with rice flour, flax seeds, and coconut oil. However, experts warn that what works for adults may not be suitable for babies, whose sensitive skin and scalp could react to the ingredients. While the ingredients may improve hair texture, they do not necessarily promote hair growth. Instead, a healthy diet and good scalp care are more important in maintaining healthy hair.

A recent consumer study has found multiple brands of soft contact lenses in the U.S. to contain "forever chemicals" that can be harmful to both the body and the environment. The study, conducted by the nonprofit organization Environmental Health Sciences, tested 18 varieties of popular contact lenses and found all of them to contain markers for PFAS. Brands such as Acuvue, Alcon, and CooperVision were among the list of affected products. This news serves as a cautionary lesson on the potential risks of overusing contact lenses.

On the birth anniversary of Dr. APJ Abdul Kalam, the ‘Missile Man’ of India, tributes pour in on social media celebrating his life, vision and impact. A visionary scientist, inspiring leader and true patriot, Dr. Kalam's humility, compassion and constant interaction with students continue to inspire generations. His tireless efforts in defense, science and youth empowerment have strengthened India's path towards self-reliance and his legacy continues to motivate young minds to dream big and work hard for the nation.

Recent studies have found that extreme heat, particularly when combined with high humidity, can have a significant impact on mental health. A study in India showed that when wet bulb temperature exceeded 27°C, the probability of reporting severe depression increased by 0.5%, even when the temperature was slightly lower. This finding is consistent with global reviews that have linked high temperatures to mood disorders, increased hospital admissions for psychiatric conditions, and even elevated suicide risk. The Lancet has also published evidence that rising temperatures worldwide are a growing threat to emotional and cognitive health.

In a meeting with university officials in Udaipur, Rajasthan Governor Hari Bhau Bagde stressed the importance of incorporating India's ancient knowledge traditions into academic research. He highlighted the deep repository of knowledge in India since ancient times and urged scholars and scientists to draw upon this tradition in their work. Bagde also suggested making ancient texts available in university libraries for study and research purposes, in order to shape the intellectual abilities and love for the nation among the younger generation.